Treating Alkalinity Problems in Aquariums and Ponds
Alkalinity is a measure of the water’s ability to buffer the pH to provide stability and avoid rapid changes in pH that could adversely affect the health of the animals in the water. It is one of the more important but most often overlooked parameters to monitor for healthy water quality in a system. Calcium carbonate (CaCO3) is the compound that has the most effect on alkalinity, but sodium, bicarbonate and other compounds play more minor roles. Good quality kits are available from companies like Salifert and Red Sea to test alkalinity by adding drops to the water sample in a test tube. Most systems will be healthiest when the alkalinity is at least 100 mg/L CaCO3. See the article on water quality for more details on specific systems.
pH is a measure of how acidic or basic is the water. Some animals need a specific range, others have a wider tolerance, but in either case drastic or rapid changes in pH are detrimental. If alkalinity is reduced, the water is subject to fluctuation in the pH and can quickly become very acidic. Alkalinity much below 50 mg/L is a very dangerous situation and could lead to pH crash, aka “Old Tank Syndrome” (see a related article in this section of the web page). This is an acute problem, but even longer term moderately low alkalinity can cause fluctuation of the pH through the day that can adversely affect health in the long term.

For those with an interest in chemistry, the buffering works by binding excess hydrogen ions. The hydrogen ion concentration determines the pH of the water:
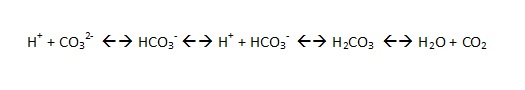
Intervention
If the system being tested is a salt water system with coral, then skip ahead to the next section, because it is far more complicated to deal with low alkalinity in that type of system.
If alkalinity is below 50 mg/L, DO NOT perform a water change until the alkalinity has been corrected. This could further reduce the alkalinity and cause a rapid drop in pH that could be fatal for many fish and invertebrates.
As stated above, calcium carbonate is the compound that has the most effect on alkalinity. Calcium carbonate can be found in coral and oyster shells. If the alkalinity is low, but not dangerously so (50-100 mg/L), then adding crushed oyster shells or crushed coral to the system can often correct the problem. They can be placed as substrate for the bottom of the aquarium, placed in a sump system or the waterfall of a pond. This will generally improve alkalinity over a few weeks, but it should be monitored every 1-2 days until over 100 mg/L.
If the alkalinity is dangerously low (below 50 mg/L) then it will need to be corrected more rapidly, especially if a water change is needed for another problem. To do this, there are many commercial products that contain buffers in a liquid form. Many are balanced calcium and alkalinity products, others only raise alkalinity. Either should be fine for fresh water aquariums or ponds as long as they are used as directed. The balanced products contain calcium carbonate. The alkalinity products often contain sodium carbonate and sodium bicarbonate. The recommended amount for the size of the system should be added at least over several hours, since the pH may fluctuate upwards with the addition of the buffer solution. This will give fish a chance to adjust slowly to changes in pH.
Reef Aquariums
The situation is more complicated in reef systems, especially those with hard coral (SPS). In these systems calcium must be considered with alkalinity to determine what steps are to be undertaken to correct the problem. In order to properly treat low alkalinity, calcium also needs to be tested. Due to the utilization of calcium and carbonate in the skeleton of the SPS coral, there is a delicate balance of the two that must be maintained. Normal calcium for an SPS system is 370-450 mg/L. With low alkalinity in a reef system, calcium may or may not also be low. If calcium is also low, then either a balanced supplement needs to be sued in order to replace both, or a calcium supplement needs to be added at the same time as the buffering solution. If calcium is in the normal range, but alkalinity is low, then a buffering solution should be added and calcium should be continued to be monitored along with alkalinity until both remain in the normal range.
Helpful hint: If calcium is continuing to be low and difficult to maintain in the normal range, then test magnesium to see if that is deficient.